Singderm® Volast Modified Dextran Gel for Injection
Caution: Only to be administered by appropriately trained healthcare professionals who are qualified or accredited in accordance with national law.
1. DESCRIPTION
Dextran................................................................90 mg/mL
Phosphate Buffer..............................................q.s. (1 mL)
This product consists of a pre-filled syringe, stainless steel needles, and gel particle encapsulated in the syringe. The gel particle is composed of self-cross-linked dextran, sodium chloride, phosphate buffer, and water for injection. The dextran is prepared through microbial fermentation. The syringe containing the gel particle has been sterilized by moist heat. The disposable sterile injection needles, crafted from stainless steel and featuring a straight sharp design, are sterilized. This product is for single use only.
2. SPECIFICATION
0.5 mL, 0.75 mL, 1.0 mL, 1.25 mL, 1.5 mL, supplied with 27G injection needles.
3. INDICATIONS
This product is applicable for injection into the middle to deep layers and subcutaneous of the facial dermal tissue to correct moderate to severe wrinkles and restore tissue volume.
4. MODE OF ACTION
This product is a filler used for dermal tissue repair and correction. By injecting cross-linked dextran gel into the dermal layer or subcutaneous tissue of hte skin, it achieves immediately visible improvement effects utilizing its physical supporting properties. Dextran can be naturally metabolized in the human body by enzymes such as glucosidase, gradually degraded into glucose monomers, and ultimately completely broken down into water and carbon dioxide for excretion. The network structure of dextran gel degrades relatively slowly in the body, allowing it to maintain long-term filling effects and improve skin condition.
5. CONTRAINDICATIONS
· The person who are allergic to any component of the product are prohibited for using.
· It is prohibited for using in patients under 18 years.
· It is prohibited for pregnant or lactating women.
· It should be used with caution for those with a tendency to keloid formation or a history of hypertrophic scar.
· Patients with atrophic skin diseases should use this product with caution.
· Those with capillary dilation should use this product with caution.
· People who are using anticoagulants such as heparin and aspirin should use this product with caution.
6. METHOD OF USE
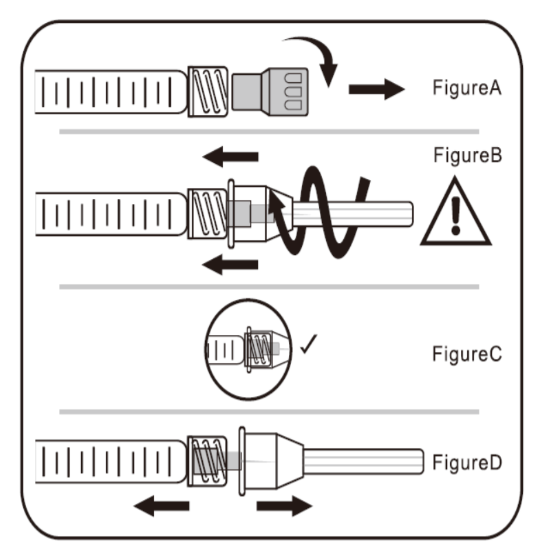
A. To attach needle to syringe
Step 1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A.
Step 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle into the luer-lock end of the syringe.
Step 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is
seated in the proper position as shown in Figure C.
Step 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needle cap as shown in Figure D.
B. Physician Instructions
1) The product must be used by healthcare professionals who have undertaken specific training in injection techniques for filling.
2) Physicians must assess whether this product is suitable for the patient and whether anesthesia is required to relieve pain. Patients should be informed of the product's scope of application, expected treatment results, precautions before and after treatment, contraindications, and possible side effects.
3) The injection should be carried out in an operating room, and the product packaging should only be opened when used in the operating room.
4) Before injection, the site must be disinfected with a bactericide.
5) Before treatment, a topical anesthetic cream can be applied to the injection site.
6) Before injection, check whether the needle and syringe are correctly installed. If abnormal resistance is encountered during injection, stop immediately and reinstall the needle.
7) Before injection, gently push the syringe to expel a small amount of air at the front end, and then the injection can begin.
8) Apply an ice pack to the injection site for 5~10 minutes after the procedure.
7. PRECAUTIONS
1) Intradermal injection carries a risk of infection, consistent with general precautions for dermal injections. Therefore, local disinfection should be performed before injection, and the injection process should follow the aseptic operation regulations of surgical treatment.
2) For patients taking medications that affect platelet function, injections may cause bruising or bleeding at the injection site.
3) Prevent mixing with any other injectable implant products.
4) The total volume of each injection should not exceed 3 mL
5) The medical effect and retention time are affected by the following factors: the nature of the defect itself, the degree of correction, the depth of implantation, the physician's injection technique, and individual physical differences.
6) Laser treatment, chemical peeling, or any other skin-irritating treatment before or after implanting this product may cause an inflammatory reaction. If the above-mentioned treatments are received, this product should not be used until the skin is completely healed.
7) Avoid washing or applying makeup to the area for 24 hours to prevent contamination. Within 7 days after the injection and implantation are completed, avoid exposing the implantation site to extreme heat (e.g., sunbathing) or cold, and refrain from skin care treatments such as massages or facial masks.
8) Do not drink alcohol or eat spicy food within one week after injection.
9) In case of any abnormal conditions after use, inform the physician immediately.
10) If injected into blood vessels, serious adverse reactions such as blindness and necrosis may occur. Therefore, be careful not to inject into arterial blood vessels, especially those around the nasolabial folds and eyes.
11) Within two weeks after the surgery, inflammatory reactions such as itching and pain caused by pressure, edema, and erythema may occur at the surgical site. These generally last for 2-3 days on average, with certain individual variations. When symptoms of redness, swelling, and edema appear, oral steroids can be taken and discontinued after the symptoms improve.
8. SIDE EFFECTS AND COUNTERMEASURES
Adverse events that may occur with the use of this product to correct wrinkles include but are not limited to: induration, itching, pain, redness, bruising, ecchymosis, infection, local inflammatory reactions, scar formation, nodules, granulomas, and allergic reactions.
Coutermeasures for Adverse Reactions
1) During the injection process, pain may occur. Due to differences in individual sensitivity thresholds and certain specific factors, such as the depth of the injection site, the proficiency of the injection technique, and postoperative handling, applying a topical anesthetic or ice - compress can be considered to improve the situation.
2) If allergic reactions such as rashes occur during the injection process, stop using the product immediately.
3) During the injection process, the skin barrier system is damaged, and infection may occur. However, if the following points are observed, infection can be avoided:
· Confirm that the packaging of the product is intact before use.
· Strictly disinfect the surgical area.
· Provide appropriate and reasonable postoperative advice and guidance to patients.
4) Slight edema and redness may occur after injection, usually disappearing within about 72 hours. The swollen area can be temporarily covered with cosmetics 24 hours after injection.
9. SHELF LIFE AND STORAGE
Shelf life is 3 years. Store at room temperature. Do not freeze.
Latest version 2000.011.02.0001 (J)-A0 lssued date: 2025-03-28


