CE Singderm® Modified Sodium Hyaluronate Gel for Injection
CE 2292
Caution: General law restricts this device to sale by or on the order of a licensed physicianor properly licensed practitioner.
BEFORE USING PRODUCT, READ THE FOLLOWING INFORMATION THOROUGHLY.
1. DEVICE DESCRIPTION
Composition (every 1ml)
Sodium hyaluronate 24mg
Lidocaine hydrochloride 3mg
Phosphate buffer pH7.2 q.s. 1ml
Singderm® is a sterile, biodegradable, nonpyrogenic, viscoelastic, clear, colorless, homogenized gel implant. It consists of modified hyaluronic acid (HA) produced by bacteria, formulated to a concentration of 24 mg/ml and 0.3% lidocaine in a physiologic buffer.
Singderm® is presented in a graduated, pre-filled, disposable syringe. Each box contains one syringe, an instruction for use, a set of labels. The contents of the Singderm® syringe are sterilized by moist heat. If presense, the needles are sterilized by ethylene oxide.
2. INTENDED USE/INDICATIONS
The product is intended to be used for mid to deep facial implantation for correction of moderate to severe facial wrinkles and folds, as well as for lip definition. The presence of lidocaine is meant to reduce the patient's pain during treatment.
3. MODE OF ACTION
Singderm® is implanted to supplement the intercellular matrix and the intradermal tissue in order to restore lost anatomical structures. Its mechanism of action is based on the latest biotechnological developments in the production of injectable hyaluronic acid. The volume and the lifting capacity originate from the ability of hyaluronic acid to attract high amount of water, which is further increased by crosslinked process. The test resuts show that the product degraded a little at 13 weeks, degraded partly at 26 weeks, and degraded completely at 52 weeks.
4.CONTRAINDICATIONS
• Singderm® is contraindicated for patients with severe allergies manifested by a history of anaphylaxis or history or presence of multiple severe allergies.
• Singderm® contains trace amounts of gram-positive bacterial proteins and is contraindicated for patients with a history of allergies to such material.
• Singderm® contains trace amounts of lidocaine and is contraindicated for patients with a history of allergies to such material.
5.WARNINGS
• The product must not be injected into blood vessels. Introduction of Singderm® into the vasculature may occlude the vessels and could cause infarction or embolization.
• Product use at specific sites in which an active inflammatory process (skin eruptions such as cysts, pimples, rashes, or hives) or infection is present should be deferred until the underlying process has been controlled.
• Do not concomitantly use disinfectants containing quaternary ammonium salts for skin preparation because hyaluronan can precipitate in their presence.
• The syring is composed by glass. Be careful in using.
• Pay attention to the physical injury caused by needles.
6.PRECAUTIONS FOR USE
• Singderm® is packaged for single-patient use. Do not resterilize. Do not use if package is opened or damaged.
• Patients should be limited to 20 mL of Singderm® per 60 kg (130 lbs) body mass per year. The safety of injecting greater amounts has not been established.
• The maximum does of the product is 8 ml for initial treatment in the face and no more than 8 ml for retreatment, and 4 ml for the initial and follow-up treatment in the lips and no more than 4 ml for retreatment; the interval between treatments is 6 months.
• As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
• Singderm® is to be used as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, homogeneity, and performance of the product and it can therefore no longer be assured.
• Singderm is intended for use in patients over 18 years of age, the safety for use during pregnancy in breastfeeding females, or in patients under 18 years has not been established.
• The safety in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
• Singderm® should be used with caution in patients on immunosuppressive therapy.
• Patients who are using substances that can prolong bleeding (such as aspirin, nonsteroidal anti-inflammatory drugs, and warfarin) may, as with any injection, experience increased bruising or bleeding at injection sites.
• After use, treatment syringes and needles may be potential biohazards. Handle and dispose of these items in accordance with accepted medical practice and applicable local, state, and federal requirements.
• Singderm® injectable gel is a clear, colorless gel without particulates. In the event that the content of a syringe shows signs of separation and/or appears cloudy, do not use the syringe.
• If laser treatment, chemical peeling, or any other procedure based on active dermal response is considered after treatment with Singderm®, there is a possible risk of eliciting an inflammatory reaction at the indications site. An inflammatory reaction is also possible if the product is administered before the skin has healed completely after such a procedure.
• Failure to comply with the needle attachment instructions could result in needle disengagement and/or product leakage at the luer-lock and needle hub connection.
• If the needle is blocked, do not increase the pressure on the plunger rod but stop the injection and replace the needle.
• Athletes should be made aware that this product contains an active principle that may produce a positive result in anti-doping test.
• Medical practitioners must take into account the fact that this product contains lidocaine.
• The composition of this product is compatible with fields used for magnetic resonance imaging.
• Serious incident associated with injection of Singderm® should be reported to the distributor manufacturer and the competent authority.
7.SIDE EFFECTS
The patients must be informed that they are potential side effects associated with implantation of this product, which may occur immediately or may be delayed. These include, but are not limited to:
• Inflammatory reactions(redness, oedema, erythema, etc.) which may be associated with itching or pain on pressure or both, occurring after the injection. These reactions may last for a week.
• Haematomas.
• Induration or nodules at the injection site.
• Staining or discolouration of the injection site.
• Poor effect or weak filling effect.
• Cases of necroses in the glabellar region, abscesses, granuloma and immediate or delayed hypersensitivity after hyaluronic acid injections have been reported. It is therefore advisable to take these potential risks into account.
• Patients must report inflammatory reactions which persist for more than one week, or any other side effect which develops, to their medical practitioner as soon as possible. The medical practitioner should use an appropriate treatment.
8.METHOD OF USE & POSOLOGY
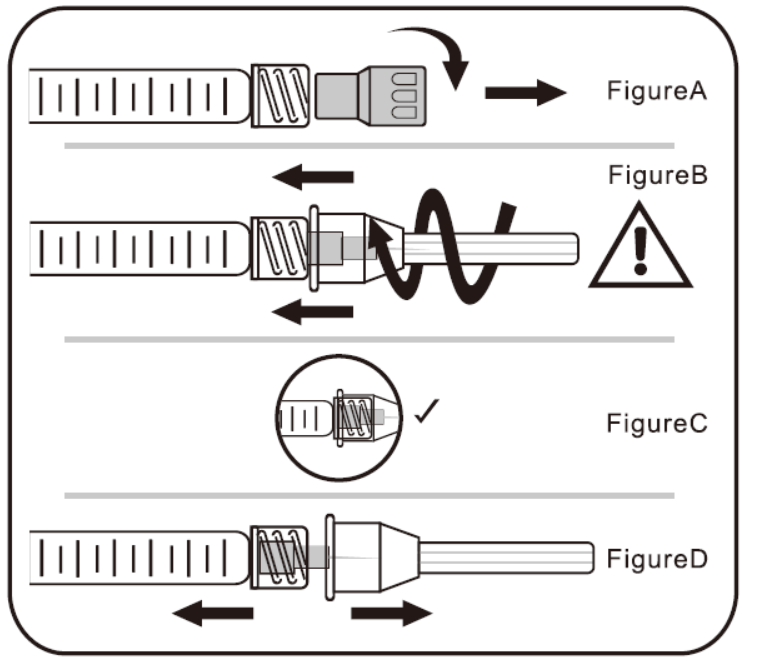
A. To Attach Needle to Syringe
STEP1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A
STEP 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle into the luer- lock end of the syringe.
For models not equipped with needles, 26G needles are recommended for 2 ml and 20-21G cannula for 10 ml.
NOTE: Needle or cannula for models other than the 1 ml equipped 27G are not part of this registration and are purchased separately.
STEP 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is seated in the proper position as shown in Figure C.
NOTE: Continue to tighten until the needle is seated in the proper position.
STEP 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needle cap as shown in Figure D
B. Physician Instructions
1) This product is designed to be injected into the dermis or the mucous membrane of the lips by an authorized medical practitioner in accordance with local applicable regulations. As precision is essential to a successful treatment, the product must be used by medical practitioners who have undertaken specific training in injection techniques for filling.
2) Before starting treatment patients should be informed of the product’s indications, contra-indications, incompatibilities and potential undesirable effects.
3) The area to be treated should be disinfected thoroughly prior to the injection.
4) Follow the above attaching needle to syringe steps, depress the plunger rod until the product flows out of the needle.
5) After the first small amount of material has been injected into the patient, wait a full 3 seconds to allow the lidocaine to take effect before proceeding with the rest of the injection.
6) The injection technique may vary with regard to the angle and orientation of the bevel, the depth of injection, and the quantity administered. A linear threading technique, serial puncture injections, or a combination of the 2 have been used to achieve optimal results. Injecting the product too superficially may result in visible lumps and/or discoloration.
7) Inject Singderm® by applying even pressure on the plunger rod while slowly pulling the needle backward. The wrinkle should be lifted and eliminated by the end of the injection. It is important that the injection be stopped just before the needle is pulled out of the skin to prevent material from leaking out or ending up too superficially in the skin.
8) If the needle is blocked, do not increase the pressure on the plunger rod. Instead, stop the injection and replace the needle.
9) The amount injected will depend on the areas which are to be corrected. Correct to 100% of the desired volume effect. Do not overcorrect. The degree and duration of the correction depend on the character of the defect treated, the tissue stress at the implant site, the depth of the implant in the tissue, and the injection technique.
10) When injection is completed, the treated site should be gently massaged so that it conforms to the contour of the surrounding tissues.
11) With patients who have localized swelling, the degree of correction is sometimes difficult to judge at the time of treatment. In these cases, it is better to invite the patient to a touch-up session after 1 to 2 weeks.
12) Patients may have mild to moderate injection-site responses, which typically resolve in a few days. If the treated area is swollen immediately after the injection, an ice pack can be applied to the site for a short period.
13) After the initial treatment, an additional treatment (from 1 to 2 weeks later) may be necessary to achieve the desired level of correction. If the wrinkle needs further treatment, the same procedure should be repeated until a satisfactory result is obtained. The need for an additional treatment may vary from patient to patient and is dependent upon a variety of factors such as wrinkle severity, skin elasticity, and dermal thickness at the treatment site.
14) The physician should instruct the patient to promptly report to her/him any evidence of problems possibly associated with the use of Singderm®.
9.Specifications
24mg/ml
1ml, with two 27Gx 1/2" TW sterile needles
2ml, without needle
10ml, without needle
10.Shelf Life and Storage
Shelf life is 2 years, Store at 2°C to 30°C, DO NOT FREEZE.
Fragile.
Latest version 8.12.04.3047 A0 lssued date: 2024-09-27
Singderm® Volast Modified Dextran Gel for Injection
Caution: Only to be administered by appropriately trained healthcare professionals who are qualified or accredited in accordance with national law.
1. DESCRIPTION
Dextran................................................................90 mg/mL
Phosphate Buffer..............................................q.s. (1 mL)
This product consists of a pre-filled syringe, stainless steel needles, and gel particle encapsulated in the syringe. The gel particle is composed of self-cross-linked dextran, sodium chloride, phosphate buffer, and water for injection. The dextran is prepared through microbial fermentation. The syringe containing the gel particle has been sterilized by moist heat. The disposable sterile injection needles, crafted from stainless steel and featuring a straight sharp design, are sterilized. This product is for single use only.
2. SPECIFICATION
0.5 mL, 0.75 mL, 1.0 mL, 1.25 mL, 1.5 mL, supplied with 27G injection needles.
3. INDICATIONS
This product is applicable for injection into the middle to deep layers and subcutaneous of the facial dermal tissue to correct moderate to severe wrinkles and restore tissue volume.
4. MODE OF ACTION
This product is a filler used for dermal tissue repair and correction. By injecting cross-linked dextran gel into the dermal layer or subcutaneous tissue of hte skin, it achieves immediately visible improvement effects utilizing its physical supporting properties. Dextran can be naturally metabolized in the human body by enzymes such as glucosidase, gradually degraded into glucose monomers, and ultimately completely broken down into water and carbon dioxide for excretion. The network structure of dextran gel degrades relatively slowly in the body, allowing it to maintain long-term filling effects and improve skin condition.
5. CONTRAINDICATIONS
· The person who are allergic to any component of the product are prohibited for using.
· It is prohibited for using in patients under 18 years.
· It is prohibited for pregnant or lactating women.
· It should be used with caution for those with a tendency to keloid formation or a history of hypertrophic scar.
· Patients with atrophic skin diseases should use this product with caution.
· Those with capillary dilation should use this product with caution.
· People who are using anticoagulants such as heparin and aspirin should use this product with caution.
6. METHOD OF USE
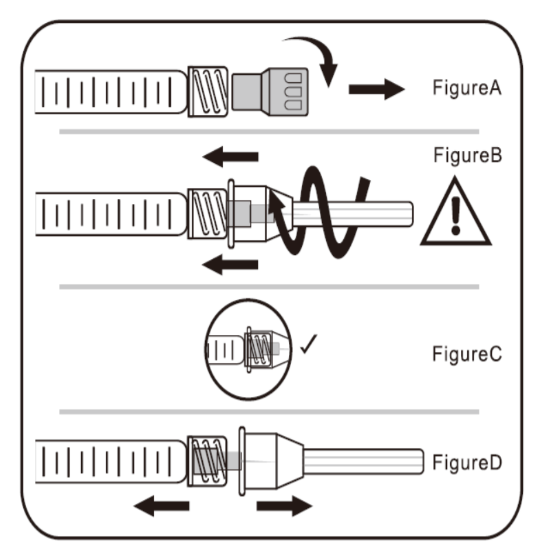
A. To attach needle to syringe
Step 1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A.
Step 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle into the luer-lock end of the syringe.
Step 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is
seated in the proper position as shown in Figure C.
Step 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite directions to remove the needle cap as shown in Figure D.
B. Physician Instructions
1) The product must be used by healthcare professionals who have undertaken specific training in injection techniques for filling.
2) Physicians must assess whether this product is suitable for the patient and whether anesthesia is required to relieve pain. Patients should be informed of the product's scope of application, expected treatment results, precautions before and after treatment, contraindications, and possible side effects.
3) The injection should be carried out in an operating room, and the product packaging should only be opened when used in the operating room.
4) Before injection, the site must be disinfected with a bactericide.
5) Before treatment, a topical anesthetic cream can be applied to the injection site.
6) Before injection, check whether the needle and syringe are correctly installed. If abnormal resistance is encountered during injection, stop immediately and reinstall the needle.
7) Before injection, gently push the syringe to expel a small amount of air at the front end, and then the injection can begin.
8) Apply an ice pack to the injection site for 5~10 minutes after the procedure.
7. PRECAUTIONS
1) Intradermal injection carries a risk of infection, consistent with general precautions for dermal injections. Therefore, local disinfection should be performed before injection, and the injection process should follow the aseptic operation regulations of surgical treatment.
2) For patients taking medications that affect platelet function, injections may cause bruising or bleeding at the injection site.
3) Prevent mixing with any other injectable implant products.
4) The total volume of each injection should not exceed 3 mL
5) The medical effect and retention time are affected by the following factors: the nature of the defect itself, the degree of correction, the depth of implantation, the physician's injection technique, and individual physical differences.
6) Laser treatment, chemical peeling, or any other skin-irritating treatment before or after implanting this product may cause an inflammatory reaction. If the above-mentioned treatments are received, this product should not be used until the skin is completely healed.
7) Avoid washing or applying makeup to the area for 24 hours to prevent contamination. Within 7 days after the injection and implantation are completed, avoid exposing the implantation site to extreme heat (e.g., sunbathing) or cold, and refrain from skin care treatments such as massages or facial masks.
8) Do not drink alcohol or eat spicy food within one week after injection.
9) In case of any abnormal conditions after use, inform the physician immediately.
10) If injected into blood vessels, serious adverse reactions such as blindness and necrosis may occur. Therefore, be careful not to inject into arterial blood vessels, especially those around the nasolabial folds and eyes.
11) Within two weeks after the surgery, inflammatory reactions such as itching and pain caused by pressure, edema, and erythema may occur at the surgical site. These generally last for 2-3 days on average, with certain individual variations. When symptoms of redness, swelling, and edema appear, oral steroids can be taken and discontinued after the symptoms improve.
8. SIDE EFFECTS AND COUNTERMEASURES
Adverse events that may occur with the use of this product to correct wrinkles include but are not limited to: induration, itching, pain, redness, bruising, ecchymosis, infection, local inflammatory reactions, scar formation, nodules, granulomas, and allergic reactions.
Coutermeasures for Adverse Reactions
1) During the injection process, pain may occur. Due to differences in individual sensitivity thresholds and certain specific factors, such as the depth of the injection site, the proficiency of the injection technique, and postoperative handling, applying a topical anesthetic or ice - compress can be considered to improve the situation.
2) If allergic reactions such as rashes occur during the injection process, stop using the product immediately.
3) During the injection process, the skin barrier system is damaged, and infection may occur. However, if the following points are observed, infection can be avoided:
· Confirm that the packaging of the product is intact before use.
· Strictly disinfect the surgical area.
· Provide appropriate and reasonable postoperative advice and guidance to patients.
4) Slight edema and redness may occur after injection, usually disappearing within about 72 hours. The swollen area can be temporarily covered with cosmetics 24 hours after injection.
9. SHELF LIFE AND STORAGE
Shelf life is 3 years. Store at room temperature. Do not freeze.
Latest version 2000.011.02.0001 (J)-A0 lssued date: 2025-03-28



