Singderm Collanat
Cross-linked Sodium Hyaluronate Gel with PLLA Microspheres
Caution: Only to be administered by appropriately trained healthcare professionals who are qualified or accredited in accordance with national law.
1. DESCRIPTION
This product is composed of a pre-filled syringe, injection needles and a gel encapsulated inside a syringe. The gel is consisted of cross-linked sodium hyaluronate (the cross-linking agent is divinyl sulfone), PLLA microspheres, non-cross-linked sodium hyaluronate, lidocaine hydrochloride, phosphate buffer system and water for injection. Sodium hyaluronate is prepared by microbial fermentation at the labeled concentration of 17 mg/mL, PPLA microspheres are at the labeled content of 16%. The labeled content of lidocaine hydrochloride is 0.3%. The injection needles are made of stainless steel and are sterilized. The syringes containing gel are sterilized with high-temperature steam. The product is for single use only.
2. INDICATIONS
Intended for deep dermis, superficial and deep subcutaneous layers injecting and filling to correct moderate and severe wrinkles
3. PRODUCT PERFORMANCE
This product is a milky white gel, with sodium hyaluronate and poly L-lactic acid microspheres as the main components. Hyaluronic acid is a unique linear macromolecular mucopolysaccharide formed by repeatedly alternating the disaccharide units of glucuronic acid and N-acetylglucosamine.
4. CONTRAINDICATION
· The person who are allergic to sodium hyaluronate, poly-L-lactic acid, lactic acid, and lidocaine hydrochloride-type local anesthetics are prohibited for using this product.
· Lidocaine hydrochloride is incompatibility with the following drugs, phenobarbital, sodium thiopental, sodium nitroprusside, mannitol, amphotericin B, ampicillin, mesopital, sulfadiazine sodium.
· It is prohibited for using in patients under 18 years.
· Patients in the early or active stage of autoimmune diseases, during immunosuppressant or immunomodulatory therapy, or with active skin infections are prohibited from using this product.
· Patients with abnormal coagulation/bleeding function, or those who have undergone treatment with thrombolytic agents, anticoagulants, or platelet aggregation inhibitors within the past two weeks, are prohibited from using this product.
· Patients with active herpes are prohibited for using this product.
· Individuals with a propensity for keloid formation or a history of hypertrophic scarring are advised to refrain from using this product.
5. WARNING
· This product is intended for single use only. Do not resterilize or reuse it.
· If the package is damaged, please do not use.
· Please check the product specifications, expiry date and batch number before use, and read the instructions carefully.
· If the needle becomes clogged, stop the injection immediately and replace the needle. Then check again to make sure there are no abnormalities, and then continue with the treatment.
· Do not use this product for injection filling in areas other than its intended application. For example, do not use it for breast filling.
· Used products and those that have exceeded their expiry date should be disposed of as medical waste.
· The container of this product is made of glass and contains sharp items such as injection needles. Be careful in using.
6. PRECAUTIONS
· Before using this product, physicians must thoroughly read this instruction manual. After understanding the product's characteristics and relating injection risks, physicians should fully communicate with patients about the potential benefits and risks associated with the treatment.
· The physician must inquire the patient's detailed medication history before treatment. If the individual is taking mediations that affect platelet function, the physician should be aware that injection procedures may potentially cause bruising at the injection site or induce bleeding reactions.
· The injection site must be sterilized before injection.
· The effectiveness and duration of treatment with this product are influenced by the following factors: the nature of the defect itself, the degree of correction, the depth of implantation, the injection technique of the physician, and individual differences in constitution. Be aware of the risks associated with swelling that may occur due to excessive use of the product. It is necessary to avoid injecting beyond the recommended dosage of the product.
· Before or after implantation of this product, undergoing laser treatment, chemical peeling, or any other skin-stimulating treatments may cause inflammatory reactions. If you have received any of these treatments, you must ensure that the skin is fully healed and verified by a medical professional before receiving this product. If you have received this product, you should wait for 1 month after the treatment and be confirmed by a medical professional before proceeding with the above treatments.
· After the procedure, it is important to maintain facial cleanliness. Within 24 hours, the injection sites should not come into contact with water, and make-up should be avoided. The injected areas should also steer clear of high temperatures and radiation, and proper sun protection should be used when outdoors.Avoid irritating drugs or foods. Avoid participating in vigorous exercise.
· After being injected and implanted into the human body, this product rarely experiences microsphere dispersion or displacement. The microspheres of this product are difficult to remove directly through surgical incision. If microsphere dispersion or displacement leads to symptoms such as tissue redness,swelling, induration, or long-term granuloma, topical or oral corticosteroid medications can be used for local anti-inflammatory treatment If necessary, surgical excision of the affected area can be considered to remove the microspheres.
· Lidocaine hydrochloride is mostly primarily metabolized by hepatic microsomal enzymes into monoethylglycinexylidide, which still possesses local anesthetic properties, and then undergoes hydrolysis by amidases before being excreted in the urine. Approximately 10% of lidocaine hydrochloride is excreted unchanged, and a small amount appears in the bile.
· Lidocaine hydrochloride may cause hypotension and bradycardia, and can lead to adverse reactions such as drowsiness, sensory abnormalities, muscle tremors, and respiratory depression.
· The safety and effectiveness of this product in combination with other drugs and devices have not been verified.
· The safety and effectiveness of this product for multiple injections have not been verified.
· The safety and effectiveness of this product in pregnant and lactating women have not been verified.
7. SIDE EFFECTS
· This product may cause adverse reactions at the injection site, including swelling, bruising, pain, and redness. These reactions can occur from a few minutes to 1~5 days after treatment. Generally, simple ice compress treatment is sufficient, and most symptoms will resolve and recover spontaneously within 1~4 weeks. If the symptoms are severed, it is necessary to consult a physician and receive clinical treatment based on the severity of the symptoms.
· Individual patients may experience infection, inflammatory reactions, or skin pigmentation at the injection site. They can consult the doctor for anti-inflammatory treatment. After the inflammatory active phase has concluded, they may also consider undergoing other relevant treatments to remove the pigmentation.
· Individual patients may experience swelling, hardening or small nodules at the injection site, which generally persist for up to 4 weeks. Small nodules may resolve spontaneously. For larger nodules, topical corticosteroid therapy can be applied locally. If nodules do not respond to medical treatment, consultation with a physician is recommended to assess the need for surgical removal.
· Appearance of symptoms of granuloma should be treated with topical or oral corticosteroids. These should be used for several weeks to months to alleviate the symptoms. This product is not suitable for individuals who have experienced similar reactions.
· Due to improper handling, the product may be mistakenly injected into a blood vessel. Such mistakes may lead to in vision, signs of stroke, whitening/necrosis of the skin in the affected area, and abnormal pain. The injection should be halted immediately and medical assistance obtained. For the sodium hyaluronate gel in the product, local injection of hyaluronidase can be administered for dissolution. For the microspheres, comprehensive treatment measures are required, including promoting vasodilation, managing skin wounds, providing nutrition, and anti-inflammatory treatment. These measures include local hot compresses, application of vasodilator drugs such as nitroglycerin, topical antibiotic ointment or oral antibiotics, and topical or oral corticosteroid therapy.
Any adverse reactions in the process of use can be directly reported to the distributor or feed back to the manufacturer by visiting http://www.singclean.net.
8. METHOD OF USE
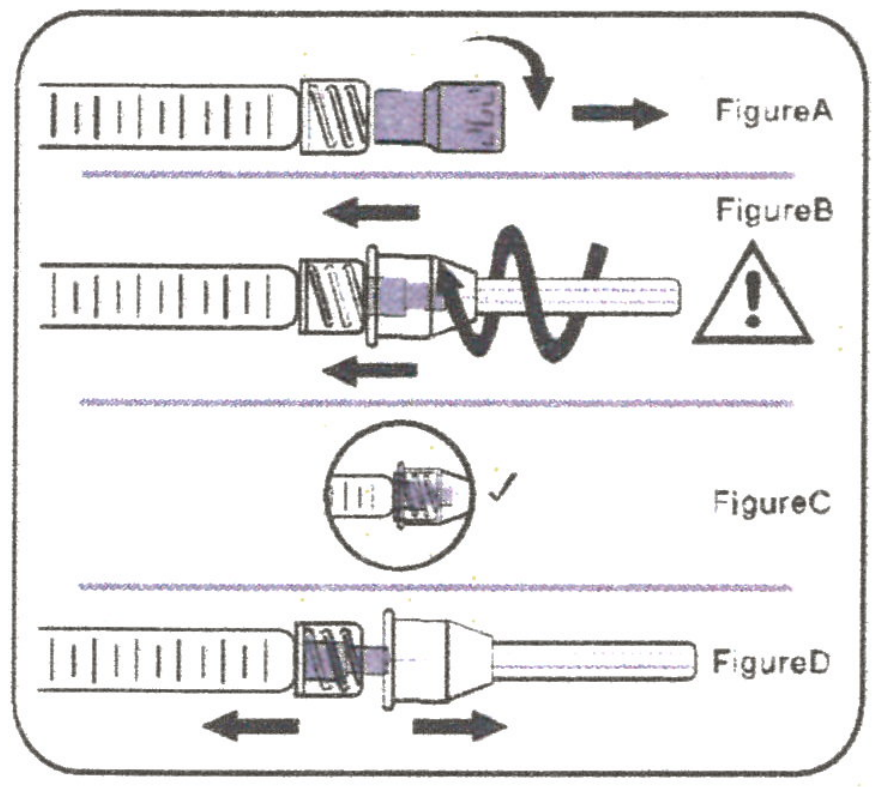
A. To attach needle to syringe
Step 1: Remove tip cap
Hold syringe and pull tip cap off the syringe as shown in Figure A.
Step 2: Insert needle
Hold the syringe body and firmly insert the hub of the needle into the luer-lock end of the syringe.
Step 3: Tighten the needle
Tighten the needle by turning it firmly in a clockwise direction (see Figure B) until it is seated in the proper position as shown in Figure C.
Step 4: Remove the needle cap
Hold the syringe body in one hand and the needle cap in the other. Without twisting, pull in opposite
directions to remove the needle cap as shown in Figure D.
B. Instructions for use
1. The suitability of the product for patient must be assessed by physician before treatment. The patient must be informed of the indications, expected therapeutic effects, precautions before and after treatment, contraindications and possible adverse reactions.
2. Keep the product at room temperature for 30 minutes before injection. Before injection, removing air from the tip of the injection needle until appears a small drop of gel.
3. This product should be injected into the deep dermis, superficial and deep subcutaneous layers of the skin. The depth and dosage of injection shall be adjusted according to the patient's condition and the physician's judgment. The total dosage of injection should not exceed 2.0 mL for a single individual. To avoid the needle entering a blood vessel, a syringe should be used to perform an aspiration test first. If blood is aspirated, the needle should be withdrawn, replaced with a new one. Then, the aspiration procedure with the syringe should be repeated. After aspirating without drawing blood, the product should be injected slowly and in small amounts. It is recommended to use multi-point injection for correction to achieve good clinical results.
4. After the treatment, the injection site can be gently rubbed to blend with the surrounding tissues, which will help to get a better correction effect. If slight swelling occurs immediately after the procedure, a short period of ice application can be used. If there is no relief, repeat the cold compress for 5~10 minutes each time.
9. SPECIFICATIONS
0.5 mL, 0.75 mL, 1.0 mL, 2x0.75 mL, 2x1.0 mL; supplied with 27G injection needles.
1.5 mL, 2.0 mL; supplied with 26G injection needles.
10. SHELF LIFE
Shelf life is 2 years.
11. STORAGE AND TRANSPORTATION
Stored at 2~20℃, not frozen.
Version: 8.26.04.0020-A1 Issued date:2025-07-30


